TiterMax® Classic Potent Vaccine Adjuvants for Clinical Trials
TiterMax® is an adjuvant used to produce cell-mediated and humoral responses in research animals. TiterMax® was developed to meet the specific needs of investigators for an immunoadjuvant that is at least as effective as Freund’s Complete Adjuvant, safer, and easier to use. It is an attractive alternative to Freund’s Complete Adjuvant for researchers to use in inducing antibodies to diverse antigens. The key to the potency of TiterMax® lies both in the immunostimulatory activity of its components and in the fact that it forms a stable water-in-oil emulsion. TiterMax® contains three essential ingredients: A proprietary block copolymer CRL-8941, squalene, a metabolizable oil, and a unique microparticulate stabilizer. Like Freund’s Complete Adjuvant, TiterMax® can be used with a wide variety of antigens because it can entrap any antigen in a water-in-oil emulsion. TiterMax® aids in the antigens’ effective presentation to the immune system without the toxic effects of Freund’s Complete Adjuvant.
History
Freund’s Complete Adjuvant (FCA) has been used for over 50 years to produce antisera in animals. The severity of its toxicity was recognized immediately, but attempts to find a less toxic and equally effective alternative have not been successful. With advances in many areas of the biological sciences and increasing concern for the welfare of experimental animals, there was increased pressure to ban or restrict the use of FCA. In 1990, we introduced TiterMax®, which has the reliability and effectiveness of FCA without the toxic side effects.
After years of research to identify and characterize the scientific basis for adjuvant activity, Dr. Robert L. Hunter led the team that developed TiterMax®. It was designed to meet the specific needs of investigators for an immunoadjuvant that is at least as effective as FCA but safer and easier to use. The key to the potency of TiterMax® lies both in the immunostimulatory activity of CRL-8941 and in its ability to form a stable water-in-oil emulsion. TiterMax® contains three essential ingredients:
- Block copolymer CRL-8941
- Squalene, a metabolizable oil
- A unique microparticulate stabilizer
Like FCA, TiterMax® can be used with a wide variety of antigens because it can entrap any antigen in a water-in-oil emulsion.
History and Structure of Copolymers
Robert L. Hunter, M.D., Ph.D., who led the team that developed TiterMax® #R-1, discovered the adjuvant action of nonionic block copolymers in the early 1980’s. The copolymers are composed of blocks or chains of hydrophilic polyoxyethylene (POE) and blocks of hydrophobic polyoxypropylene (POP) attached in one of four configurations (Figure 1). By varying the size of the blocks of POE and POP, dozens of preparations have been produced that span nearly the entire range of functional activities of available nonionic surfactants. The new copolymer CRL-8941 was developed specifically by CytRx Corporation to optimize the structural features essential to adjuvant activity while minimizing the inflammatory properties. The rationale for the synthesis of CRL-8941 evolved from the studies of Hunter and associates on the physicochemical parameters that contribute to adjuvant activity. These properties of such adjuvants are summarized here.
In initial studies with triblock copolymers, one of the preparations (L121) was found to serve as an adjuvant for stimulating antibody formation to BSA following injection in an oil-in-water emulsion. A second copolymer (L101) stimulated little antibody but was more effective in inducing DTH and granulomas. The other triblock copolymers available at that time had lower adjuvant activity for either type of response. This demonstrated that small changes in the lengths of chains of POP and POE produced major changes in biological activity and provided the foundation for further studies.
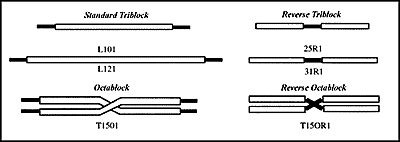
Figure 1. Structure of Block Copolymers.
The copolymers are composed of blocks of hydrophilic polyoxyethylene (POE, black) attached to blocks of hydrophobic polyoxypropylene (POP, white). The diagrams are drawn to scale for both length and thickness. Only one or two copolymers from each group are shown, but each copolymer family contains a series of members that differ in the length and relative proportion of POE vs. POP.
Adhesion and Adjuvant Activity
Hydrophile-lipophile balance (HLB) is a measure of the relative strength of the hydrophilic and hydrophobic activities of nonionic surface active agents, and it has been widely used to predict functional activities. The copolymers and other surfactants with adjuvant activity were found to have HLB values of less than two and were classified as spreading agents. Spreading agents are insoluble in water and do not emulsify or solubilize lipids or membranes. They are very different from common detergents, which have HLB values greater than ten. They adhere to lipids and influence the interaction of soluble macromolecules with them. Hunter and associates demonstrated a correlation between the ability of copolymers to promote the retention of soluble macromolecules on the surface of oil drops and their activity as adjuvants. The local concentration of antigen fixed to a surface is far higher than can be achieved by comparable amounts of material free in solution. Thus, the copolymers, which were adjuvants, produced a concentrated surface matrix that facilitated antigen presentation to cells of the immune system.
Copolymers that differ only in the lengths of chains of POP and POE demonstrate a great variety and intensity of immune and inflammatory responses. When a series of copolymers with similar low HLB values but differing in the size of the component blocks and their mode of linkage was tested, several patterns of immune response and inflammation were noted. The most effective adjuvants were the largest copolymers with hydrophilic POE on the ends (L121, L101, and T1501). Increasing the length of the POE chains even modestly caused the preparations to lose their activity as adjuvants. Shortening the length of the POP chains produced agents with lower adjuvant activity and greater toxicity as measured by inflammation at the site of injection. Placement of POE in the reverse configuration (at the center of the molecule flanked by POP) produced weak adjuvants, which stimulated granulomatous inflammation. Interestingly, the intensity of the inflammatory responses did not correlate with antibody titers.
Analysis of the surfaces formed by selected copolymers and their interaction with plasma proteins provided interesting information (Figure 2). The copolymers all spontaneously form highly organized structures at an aqueous surface, and the heterogeneity of these structures contributes to the heterogeneity of their biological activity. Thus, the copolymers, which are adjuvants, form a particular type of hydrophilic adhesive surface. If the blocks of POE are too long, the surface is not sufficiently adsorptive. If the blocks of POP are too short, they are unable to fold to produce a hydrophilic surface.
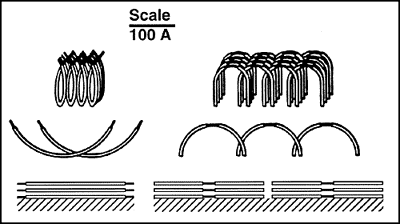
Figure 2. Model of Surfaces of Copolymers Spread at Varying Concentrations.
Triblock copolymer L121 and reverse triblock copolymer 31R1 are drawn to scale with the POE portions black and the POP white. At low surface coverages, both copolymers lie flat, producing largely hydrophobic surfaces (POP exposed). As the amount of copolymer on the surface increases, the hydrophobicity decreases, demonstrating that the molecules become arranged with the POE oriented towards the surface. The surface of thick layers of L121 consists entirely of POE. It is hydrophilic and permits the underlying organizational tendencies of the copolymer to be expressed as fibers. The reverse copolymers cannot form hydrophilic surfaces because their POE portions are sterically inhibited. This accounts for their formation of spherical oil-like drops in saline. These orientations account, at least in part, for the biological activity of the various copolymers.
Biologic Activities of Copolymers
Subsequently, it was found that copolymers with adjuvant activity not only bind antigens but they activate macrophages as well. The ability to activate macrophages, however, was not sufficient to produce adjuvant activity. The antigen binding capacity of a given copolymer shows synergy with its potential to activate macrophages, suggesting that enhancement of antigen-presenting capacity also plays a role in copolymer adjuvant activity. Finally, the adjuvant copolymers were shown to activate complement. This probably influences the localization and retention of antigens in lymphoid tissue and the activation of immunoreactive cells.
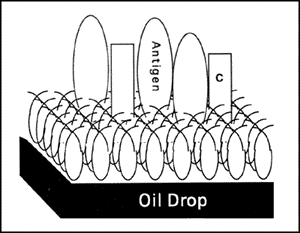
Figure 3. Model of an Immunogenic Particle.
The copolymer folds to form a hydrophilic adhesive surface. Antigen (BSA), complement (C) and mediators bound to the surface are presented to cells of the immune system in a particularly concentrated and effective form.
A model of an active adjuvant preparation is shown in Figure 3. The surface of an insoluble polymer that concentrates antigen and activates complement and other mediators would be expected to provide a powerful immunogenic stimulus. The hydrophobic surface of the oil drop is necessary to support the copolymer. The low free energy (hydrophilicity) of the surface allows the generation of a large surface area. The geometry of the molecules and their balance of hydrophilic and hydrophobic properties determine the adhesive properties. Variation in adhesive properties of the copolymers affects the concentration and conformation of adherent proteins, and this, in turn, influences the type and intensity of biological response. The copolymer, which is in TiterMax® #R-1, CRL-8941, was specifically tailored to optimize the physical-chemical properties that promote adjuvant activity. The other components of the TiterMax® #R-1 formulation function to produce a stable emulsion that insures effectiveness with a wide variety of antigens.
What Is Special About TiterMax® Emulsions?
The physical form of the adjuvant emulsion is important. In our early experiments, when lyophilized antigen was incorporated into oil drops by homogenization with the hydrocarbon and copolymer before adding saline, the adjuvants were effective. When the antigen was first dissolved in saline, the adjuvants frequently failed. Again, there was a correlation between the adhesion of antigens to the adjuvant and the success of immunization. If the antigen was physically incorporated into oil drops, then a strong primary antibody response was usually achieved. This response could be boosted to very high levels and frequently persisted for over one year. If the antigen was not incorporated in the oil drops, then the response depended on adhesion to the surface. Antigens that adhered strongly worked well, but most antigens failed to induce primary or lasting secondary responses. Since preparation of optimally effective oil-in-water immunogens is difficult with all antigens and impossible with many, we invested effort to develop TiterMax®, an adjuvant that could readily form water-in-oil emulsions with a wide variety of aqueous antigens usually used by the research scientist. It is stabilized with a particularly effective copolymer adjuvant and emulsion-stabilizing microparticles.
Physical contact between antigen and adjuvant is an important component of the activity of most adjuvants. Exceptions are primarily limited to particulate antigens to which the response can be boosted by agents that induce inflammation or stimulate cytokines. Oil emulsions facilitate sustained contact between antigen and adjuvant while their form and stability contribute strongly to their effectiveness for immunization. Emulsions, mixtures of two immiscible fluids, one of which is suspended as small drops inside the other, are stabilized by surface active agents. There are two principal kinds of emulsions: water-in-oil and oil-in-water. In the former, oil forms the continuous phase, which surrounds small droplets of water, or the discontinuous phase. Water forms the continuous phase in an oil-in-water emulsion. Both types of emulsions have been used as adjuvants. Water-in-oil formulations, such as TiterMax® #R-1 and Freund’s, are the most powerful adjuvants in most protocols. They induce higher, longer-lasting titers with fewer injections and frequently require lower doses of antigen.
TiterMax® #R-1 has been specially formulated to maximize the desirable effects of water-in-oil emulsions while minimizing or eliminating the undesirable side effects of FCA. It consists of a metabolizable oil, squalene, with emulsifiers that facilitate the production of water-in-oil emulsions with up to 90% water. This means that an injection of TiterMax® may contain less oil than is commonly used in less effective oil-in-water emulsions. TiterMax® is easier to emulsify than Freund’s adjuvant. The resulting emulsion is less viscous, making it easy to inject through small needles.
Stable water-in-oil emulsions are notoriously difficult to make. Interestingly, they can be stabilized by incorporating microparticles. Depending on whether the particles are hydrophobic or hydrophilic, they will facilitate the production of an oil-in-water or a water-in-oil emulsion. The most stable emulsions are obtained when the contact angle with the solid at the interface is close to 90°, the type depending on whether the angle is greater or less than 90°. A concentration of solids at the interface forms a very strong and stable interfacial “film,” which stabilizes the emulsions. Thus, we have incorporated microparticles into TiterMax® adjuvant with surface properties that facilitate the formation of highly stable water-in-oil emulsions with aqueous antigens. These particles make it possible to form stable emulsions with some surface active antigens, which break normal emulsions. They also contribute significantly to adjuvant activity. A model for the TiterMax® emulsion is shown in Figure 4.
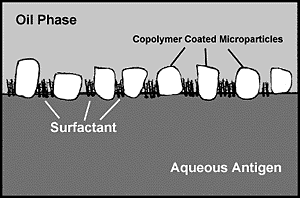
Figure 4. Model of Microparticle Stabilized Water-in-Oil Emulsion.
Microparticles potentiate emulsion stability and adjuvant activity while reducing the requirement for surfactants. Their localization at the interface between oil and aqueous compartments provides increased physical separation of adjacent droplets, retards the surface area, which must be stabilized by surfactant, and reduces interfacial free energy. The orientation of the copolymer CRL-8941 on the surface of the microparticles is critical. It provides surface properties ideal for stabilizing water-in-oil emulsions. It also contributes to the expression of adjuvant properties by displaying the copolymer in a condensed fashion on bonded surfaces.
The TiterMax® formulation was developed to produce very stable emulsions, but the initial preparation of the emulsion is still highly dependent upon the technique. Although TiterMax® emulsions can be prepared using any technique that works with Freund’s adjuvant, we recommend one of the procedures described in detail in the instruction sheet for TiterMax®. Procedures are available for the well-known two-syringe method, a micro method for small volumes of antigen, as well as sonication and homogenization. The choice of method will depend on the equipment available and the amount of emulsion to be prepared. Transformation of your antigen/TiterMax® mixture into a water-in-oil emulsion can be tested by placing a drop on water.
Immunogens which contain high concentrations of surfactants or other materials may interfere with emulsification. We have found that SDS, which may be present in acrylamide gels, in concentrations greater than 1% or urea concentrations greater than 1.0 M significantly reduce the emulsifying capacity of TiterMax®. Other similar materials are likely to have the same effect. Some surface active agents serve as demulsifying agents that break emulsions. The modern emulsifiers and microparticulate stabilizers of TiterMax® are able to overcome the effects of most such agents present in moderate quantities. Glutaraldehyde does not affect the emulsion. TiterMax® is compatible with organic compounds and solvents such as DMF in moderate amounts.
Many of our customers ask about storing TiterMax® emulsions. A 50:50 water-in-oil emulsion can usually be stored at room temperature, 4° C, -20° C, or -70° C, for as long as the antigen is stable. Upon storage, approximately 20% of the oil will dissociate from the emulsion. You may leave the emulsion in a syringe and simply reemulsify when ready to inject (provided the antigen is stable). TiterMax® emulsions have been repeatedly frozen and thawed or left at room temperature for several weeks with no loss of efficacy. Sodium azide or thimerosal, which are present as preservatives in some antigen preparations, will not affect the shelf life of TiterMax®, although at high concentrations of either, the emulsion could become toxic.
Examples of Successful Dosing Regimens for TiterMax® Classic
| Species | Injection Route | Total Injections | Volume Per Injection | Site(s) |
| Mice | IM | 2 | 20 ul | Each Hind Quadricep |
| SC | 1 | 40 ul | Base of Tail | |
| Rats | IM | 2 | 50 ul | Each hind quadricep |
| Guinea Pigs | IM | 2 | 50 ul | Each hind quadricep |
| SC | 4 | 50 ul | Over both shoulders and both hind quadriceps | |
| Rabbits | IM | 2 | 40 ul | Each hind quadricep |
| SC | 4 | 100 ul | Over both shoulders and both hind quadriceps | |
| ID | 10 | 40 ul | Along back | |
| Chickens/Turkeys | IM | 2 | 50 ul | Each breast |
| SC | 1 | 100 ul | Neck | |
| Cats/Dogs | IM | 2 | 125 ul | Each hind quadricep |
| SC | 1 | 125 ul | Along neck | |
| Rhesus Monkeys | IM | 2 | 100 ul | Each hind quadricep |
| SC | 4 | 50 ul | Along inner thigh | |
| Goats/Sheep | IM | 4 | 250 ul | 2 injections in each hind quadricep |
| Cows/Horses | SC | 10 | 100 ul | Along neck |
Good immune responses have been achieved with Water to TiterMax® Classic ratios of 50:50 to 90:10; however, the 50:50 water-in-oil emulsion is usually optimum.
*These are suggested routes and dosages that have proven successful and then reported to TiterMax USA, Inc. by numerous investigators. Dosing regimens that have been used with other adjuvants may certainly use TiterMax® Classic.


